Introduction
The long-term survival for children with AML has increased to 70 % - but relapsed AML patients still have a poor prognosis (about 30 %).
Recent data suggest that in AML residual leukemic cells below the 5 % limit required for a morphologic CR can adversely affect outcome and that increasing levels of Minimal Residual Disease (MRD) are almost always followed by clinical relapse. Early MRD detection provides an opportunity to implement pre-emptive therapies as a bridge-to-transplant approach, to potentially prevent hematological relapse and increase the probability of successful HSCT. A promising approach is the use of Azacitidine (Aza) for treatment of molecular relapse which we evaluated within the clinical trial “AMoRe2017”. Aza has been shown to reverse epigenetic changes in malignant cells, restoring the normal function of genes critical for differentiation and cell cycle control. The trials' primary objective was the evaluation of the effect of Aza treatment in AML subjects at molecular relapse after CR1 regarding molecular response prior to further treatment (reinduction/HSCT).
Patients and Methods
AMoRe2017 enrolled children with AML (N=20), >3 months < 21 yrs., of which n=1 dropped out due to laboratory measurement error. The median age was 10.9 yrs. (range 1.7-18.9 yrs.). N=10 (53 %) had a CBFB-AML, n=5 (27 %) KMT2A-rearrangement, n=1 (5 %) NUP98::NSD1, n=1 (5 %) FLT3-ITD, n=1(5 %) PICALM::MLLT10 and n=1 (5 %) NPM1. N=8 (42 %) were classified to subgroup M2, n=4 (21 %) to M5, n=3 (16 %) into M1 and n=4 (21 %) to M4/M4 Eo. At baseline, the white blood cell count averaged 3.8x10 9/l (range 0.1-6.6x10 9/l), without leukemic blasts in PB or BM.
Each patient had at least one quantifiable genetic marker (rt-PCR), achieved CR with molecular remission, following initial therapy, and subsequently had a molecular relapse (defined as an increase of the MRD level by at least 1 log, less than 5 % BM myeloblasts and absence of proven extramedullary disease). Patients were treated for up to 6 cycles with 75 mg/m 2 i.v. Aza on days 1 to 7 in a 28-day cycle.
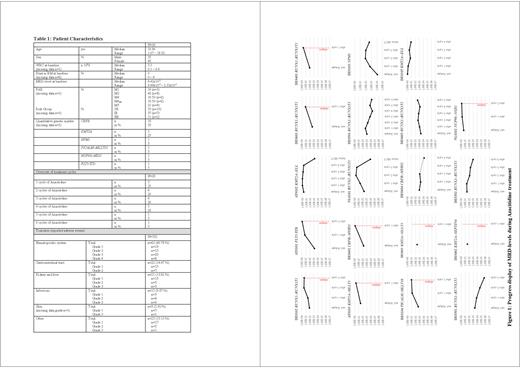
Molecular response was categorized as molecular stabilization (< 1 log decrease or increase from baseline), molecular progression (≥ 1 log increase from baseline without hematological relapse), molecular improvement (≥ 1 log decrease from baseline), and hematological relapse (at least 5 % blasts in PB and/or BM and/or proven histological extramedullary relapse).
Results
During Aza treatment, 10 patients (53 %) had an open relapse, which occurred in n=9 after 1 st or 2 nd cycle. These patients were monitored for KMT2A::SEPTIN6 (n=1), KMT2A::MLLT3 (n=2), PICALM::MLLT10 (n=1), FLT3-ITD (n=1), NUP98::NSD1 (n=1), CBFB::MYH11 (n=1) and RUNX1::RUNX1T1 (n=3).
9 patients (47 %; RUNX1::RUNX1T1 n=5, CBFB::MYH11 n=1, NPM1 n=1, KMT2A::ELL n=2) showed a molecular response to Aza and proceeded to HSCT without receiving intensive re-induction treatment.
16 out of the 19 patients are alive (overall survival 84 %), 2 died from HSCT-associated side effects (VOD, infection), and 1 from disease progression.
Considering genetics, 7 out of 11 patients with favorable genetics (core-binding leukemia n=10 or NPM1 n=1) responded to the Aza therapy ( RUNX1::RUNX1T1 n=5, CBFB::MYH11 n=1, NPM1 n=1).
By contrast, in cases with KMT2A-rearrangements, NUP98::NSD1 or FLT3-ITD, 2 out of 8 patients showed improvement or stabilization.
In total 152 adverse events (AEs) associated to Aza were reported, the majority classified as grade 1 or 2 (n= 114; 75 %). Mainly affected was the hematopoietic system (40 %), the gastrointestinal tract (15 %), kidney and liver (14 %). Cardiotoxicity was not reported.
Discussion
The use of Aza as pre-emptive “bridge-to-transplant” approach treating the molecular relapse showed efficacy in patients with RUNX1::RUNX1T1, CBFB::MYH11, NPM1, and KMT2A::ELL, who could be transplanted directly. This pilot provided proof of principle that molecular relapses could be treated, and MRD levels be reversed, at least in subgroups. In addition, avoiding intensive re-induction therapy prior to HSCT is feasible.
Conversely, the relatively slow-acting mechanism of epigenetic therapy seems to be ineffective in highly proliferative subgroups of AML with KMT2A-rearrangements or FLT3-ITD.
Further studies are mandatory to define treatment options for molecular relapses of non-favorable pediatric AML and to confirm potential improvement of outcome post-HSCT.
OffLabel Disclosure:
Reinhardt:Cerus: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusa: Speakers Bureau; Medac: Consultancy, Research Funding, Speakers Bureau; MSD: Speakers Bureau; BluebirdBio: Research Funding, Speakers Bureau; Immedica: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; JAZZ Pharmaceutical: Research Funding. Zwaan:Sanofi: Other: Advisory role; BMS: Consultancy; Gilead: Consultancy; Syndax: Research Funding; Abbvie: Research Funding; Kura Oncology: Consultancy; Novartis: Consultancy, Other: Advisory role; Jazz Pharmaceutical: Research Funding; Abbvie: Research Funding; Takeda: Research Funding; Pfizer: Consultancy, Research Funding; Incyte: Consultancy; ITCC Hem Malignancies Committee: Membership on an entity's Board of Directors or advisory committees.
Cytidine analogues such as azacitidine were shown to induce cell differentiation and to inhibit the methylation of newly synthesized DNA. Azacitidine is used in pedaitric AML to treat molecular relapse.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal